Experiments with water, pressure and temperature
If you were on a mountain top you would find the water in a kettle would start boiling at less than 100 °C.
Why? Because the air pressure is much lower the higher up you are – and the lower the pressure the lower the temperature at which liquids boil.
You can change the temperature at which any liquid boils by changing the pressure. Boiling at a particular pressure is known as Vaporization or Evaporation.
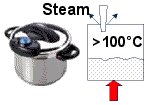
Pressure Cooker
You can speed up the cooking times for some foods by using a pressure cooker. The lid on the machine is sealed tight and the increase in pressure as the liquid heats causes the water to boil at a temperature of more than 100°C.

Vaporization or Evaporation?
We use the terms Vaporization or Evaporation instead of boiling, because this is a bit more technical and refers to boiling at a particular pressure. We say the evaporating temperature of a fluid at a certain pressure is xx °C.
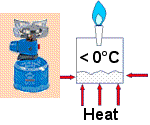
In the camping gas stove propane is stored under pressure as a liquid. When the valve is opened the pressure drops and the liquid starts to boil (sorry, evaporate!). Where does the heat come from to boil the propane? It comes from the surrounding air, and because the propane is evaporating at a low temperature, the outside of the can becomes cold. The liquid is drawing in heat to enable it to boil. The can cools down the surrounding air. That's good - we've made the first step to building our fridge!
All we have to do now is to catch the gas, re-pressure it, and turn it back to a liquid. This is How Your Fridge Works too.
Below is a thermal imaging camera measuring the temperature of a torch used to caramelise sugar when you are cooking. You can see how the temperature of the cylinder that contains the gas drops as the flame burns. It starts at 18.5 degrees and goes down to 17.4 by the end of the video.