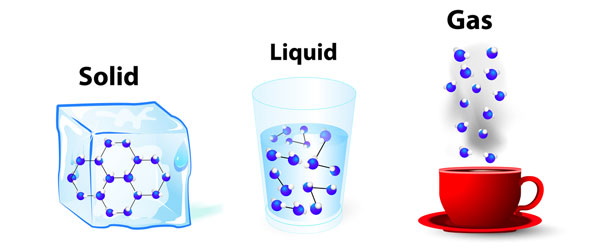
What is happening when a solid becomes a liquid or a gas?
Substances are made of molecules. In a solid the molecules are packed tightly together, in a liquid the molecules move around more energetically and in a gas there is a lot of space between molecules and they flow around freely. When a substance is heated the molecules start to move around more, they take up more space and the substance expands. It is not the molecules themselves that get bigger, but the room they need to move around. The heat that is applied provides the molecules with the energy they need to increase their movement.
In the same way, if things are cooled energy is extracted as heat, and eventually the substance wil go through a phase change from gas to liquid to solid.
A good example of this occurring naturally is steam condensation as water on a cold surface in a bathroom. The warm steam meets the cold mirror surface, which conducts the heat of the steam away and liquid water droplets are formed.
In your fridge the heat is extracted by a liquid changing state to a gas - this substance is known as a refrigerant. Inside the refrigerator there is a continuous circuit that allows the refrigerant gas to be turned back into a liquid and recirculated.
Try our phase change game to test your knowledge
